Introduction
Over the next three weeks, we will begin a process known as molecular cloning. Molecular cloning uses the machinery of cells (such as bacteria) to produce a large number of copies of a short sequence of DNA. It is not the same as the cloning of organisms where multiple individuals with identical genomes are produced. Unless otherwise noted, in this course, "cloning" will refer to molecular cloning.
Cloning vectors
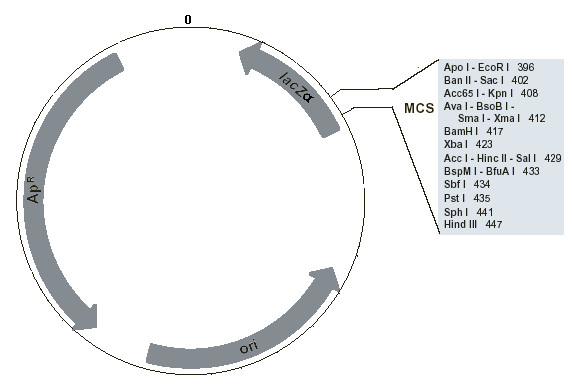
Fig. 5. Location of important features in the pUC19 cloning vector plasmid. ApR is the ampicillin resistance gene, lacZα is the lacZ gene, and ori is the origin of replication. The numbers represent base pairs measured from an arbitrary reference point on the vector, labeled 0. The basepair numbers increase clockwise until the reference point is reached again after 2686 bp. Image derived from New England Biological technical information
Central to the cloning process is the cloning vector. A cloning vector is a self-replicating sub-cellular entity that can take up foreign DNA, infect a host cell, and multiply. Bacterial plasmids are commonly used cloning vectors for small DNA fragments and the bacteriophage lambda (a virus) is used for larger fragments of approximately 15 kbp. We will use a plasmid known as pUC19 as the vector in our cloning experiment. pUC19 originated as a "wild" plasmid that occurred naturally in bacteria, but its DNA sequence has been heavily engineered to add several features that make it useful as a vector (Fig. 5). Each of the features serves an important purpose in allowing the plasmid to replicate and survive the screening processes used in molecular cloning.
Inserting foreign DNA

Fig. 6 Ligation of an insert into a plasmid vector
A plasmid is a closed circular piece of double-stranded DNA. Plasmids are relatively small (pUC19 has 2686 bp) in comparison to the millions of basepairs in a prokaryotic genome or the chromosome of a eukaryote. When it is placed into a bacterium, the cell's machinery is used to replicate the plasmid, starting at a position known as the origin of replication (ori). Depending on the type of plasmid and condition of the cell, the plasmid can rapidly achieve a high copy number within the cell. When a plasmid is used as a vector, the circular DNA is cut and a foreign fragment of DNA, known as an insert, is ligated (attached) into the circle at the point of the cut (Fig. 6) .The combined circle of DNA is called a recombinant plasmid consisting of two parts: the vector (most of the original plasmid) and the insert (the foreign fragment). The insert is then mass-produced when the recombinant plasmid is replicated inside a bacterium (Fig. 7).

Fig. 7 Replication of a recombinant plasmid in a bacterium
In this series of experiments, you will clone one of three unknown DNA fragments. Each fragment was obtained from a research lab in the VU Medical Center and consists of the major part of a gene that is important in cancer research. One of your tasks will be to use data that you have collected to identify which of the three genes is your unknown.
Why clone?
Originally, cloning was the only way to produce large amounts of segments of DNA. PCR (polymerase chain reaction) is now a faster and easier method of producing large amounts of short DNA sequences. However, before PCR can be used, appropriate primers must be created. This requires some knowledge of the sequence that may not be available when studying a new gene. Therefore, cloning is still the method of choice in the search for an unknown gene. By randomly cloning all of the segments of the target organism's genome, a genomic library can be created with each segment present in a separate bacterial colony. A labeled mRNA probe that will bind with the gene of interest can be used to screen the library to identify which colony contains the gene. Bacteria from this colony can subsequently be isolated and cultured to provide a source of DNA containing the gene.
Serial cloning is a commonly used method for splicing together fragments from several genes. When the recombinant gene is placed back into the organism, it can have new functions not present naturally. For example, parts of the gene for green fluorescent protein from jellyfish have been inserted into genes from other organisms. When the other gene is turned on, the tissues in which the gene is expressed glow green. Microscopic observation allows the researcher to identify those tissues and infer the temporal and spatial patterns of gene expression.
Insertion of genes into expression vectors is another important use of cloning. Some proteins are present in extremely tiny quantities in the cells of living organisms. This makes it very difficult to extract enough of that protein to study. If the gene for that protein is inserted into an expression vector, the bacteria carrying the recombinant expression vector will produce the protein in large amounts, making it relatively easy to extract and purify as much protein as desired.
Preparing the pUC19 vector for an insert
As discussed previously, a closed circular cloning vector plasmid must be cut open before a DNA fragment can be inserted for cloning. This cut cannot be made randomly because certain parts of the vector have important functions that would be disrupted if foreign DNA were inserted there. pUC19 and most other plasmid vectors have a specially engineered stretch of DNA, called the multiple cloning site (MCS, also known as a polylinker).
The MCS of pUC19 (Fig. 5) consists of a 54 bp sequence containing recognition sites for several common 6-cutter restriction endonucleases (Fig 8).

Fig. 8 Sequence of the pUC19 multiple cloning site. From NEB technical information
An important feature of any MCS is that the recognition sites are unique to the MCS. In other words, any restriction endonuclease for which a recognition site is included in the MCS will cut only at that site and nowhere else in the vector sequence.
There are two main methods for inserting DNA in an MCS. The vector can be cut at the MCS with a single restriction enzyme (a single digest). A DNA fragment cut on both ends with that same restriction enzyme can then be ligated into the MCS because its sticky ends will be complementary to those at the MCS cut. However, during the ligation step, many of the vector molecules will close again without taking up an insert. To minimize this, the ends of the vector can be dephosphorylated, allowing them to ligate with the insert but not with each other. Another problem with the single digest method is that it is not possible to control the orientation of the fragment when it is ligated into the MCS of the vector, because both ends have the same overhanging sequence. In some cases, the fragment will be inserted one way and in others it will be reversed.
A second method, known as directional cloning, overcomes these problems. In directional cloning, the vector is cut with two of the restriction enzymes that have recognition sites in the MCS. This produces two vector fragments: a tiny piece that consists of the part of the MCS between the two restriction sites, and a large piece that contains most of the vector. Because each of these pieces do not have two ends that are complementary, neither of them can close upon themselves. The original non-recombinant circular vector can only be recreated if the tiny piece is re-ligated into its original position in the MCS. This can be prevented by removing the tiny piece through gel purification. This extra step can be avoided in certain plasmids (such as pUC19) through a screening method that allows the researcher to differentiate between recombinant plasmids that successfully took up the foreign DNA insert and non-recombinant plasmids in which the tiny fragment was reinserted in the MCS.
In directional cloning, the foreign DNA fragment to be inserted must be cut with the same two restriction enzymes that were used to cut the vector. This gives the insert two ends that are complementary to the two ends of the vector. Because the two ends of the insert are different, they can only be oriented one way to be successfully ligated into the MCS of the cut vector.
In this experiment, we will prepare the pUC19 vector plasmid for use in directional cloning by digesting it with two restriction enzymes. The two enzymes will depend on the unknown that you have been assigned. All of the unknown fragments were cut on one end with EcoR I. Unknowns 1 and 2 were cut on the other end with BamH I and Unknown 3 was cut on the other end with Xba I. So students who have Unknowns 1 and 2 will digest their pUC19 with EcoR I and BamH I, while students having unknown 3 will digest their pUC19 with EcoR I and Xba I.
